Some foreign medical device manufacturers are surprised when they learn that the device classification rating they received in their home country is not the same rating that they receive in China. In fact, China’s medical device classification system is oftentimes stricter than abroad.
In this post, we review China’s classification system for medical devices to help you determine what class your specific device will receive.
Lastly, we go over some of the additional requirements your device will need to overcome if you are planning to register a class II and III medical device.
What Determines Device Classification In China?
Just like in foreign countries, China’s medical device classification system is based on the degree of risk associated with that product.
The higher the risk, the higher the class category. According to China’s NMPA, the degree of risk is determined by:
- Intended purpose
- Structural & material characteristics
- How it is used
- If the device is body contacting
China’ medical device classification system is broken down into three classes.
Class I Devices
This includes low-risk medical devices with a high degree of safety and effectiveness. These devices are not subjected to technical reviews by the NMPA and only need to comply with the minimum regulatory requirements.
- Home country test reports OK
- Timeline: 10-15 Months
- Expiry: None
- Application Fee: None
Class II Devies
These devices are considered medium-risk and moderate measures are required to ensure safety and effectiveness. Typically, a submission of the device’s full registration dossier will be required, including research, validation, and verification documentation.
- Additional In-Country Tests Required
- Must Meet Chinese Standards
- Timeline: 18-24
- Expiry: 5 years
Class III Devices
These devices are considered high-risk and considerable measures are required to ensure safety and effectiveness. Typically, a submission of the device’s full registration dossier will be required, including research, validation, and verification documentation.
- Additional In-Country Tests Required
- Animal & Clinical Trials Likely
- Must Meet Chinese Standards
- Timeline: 18-24
- Expiry: 5 years
Summary
| Device Class | Registration Type | Review Type | Registration Timeline | Validity |
|---|---|---|---|---|
| Class I | Notification | Administrative Review | 3-6 Months | Unlimited |
| Class II | Registration | Full Application Review | 24+ Months | 5 Years |
| Class III | Registration | Full Application Review | 24+ Months | 5 Years |
NMPA Device Classification Table
Refer to the below table to determine the class level for your medical device in China.
Table 1: Body Contacting & Non-Active Devices
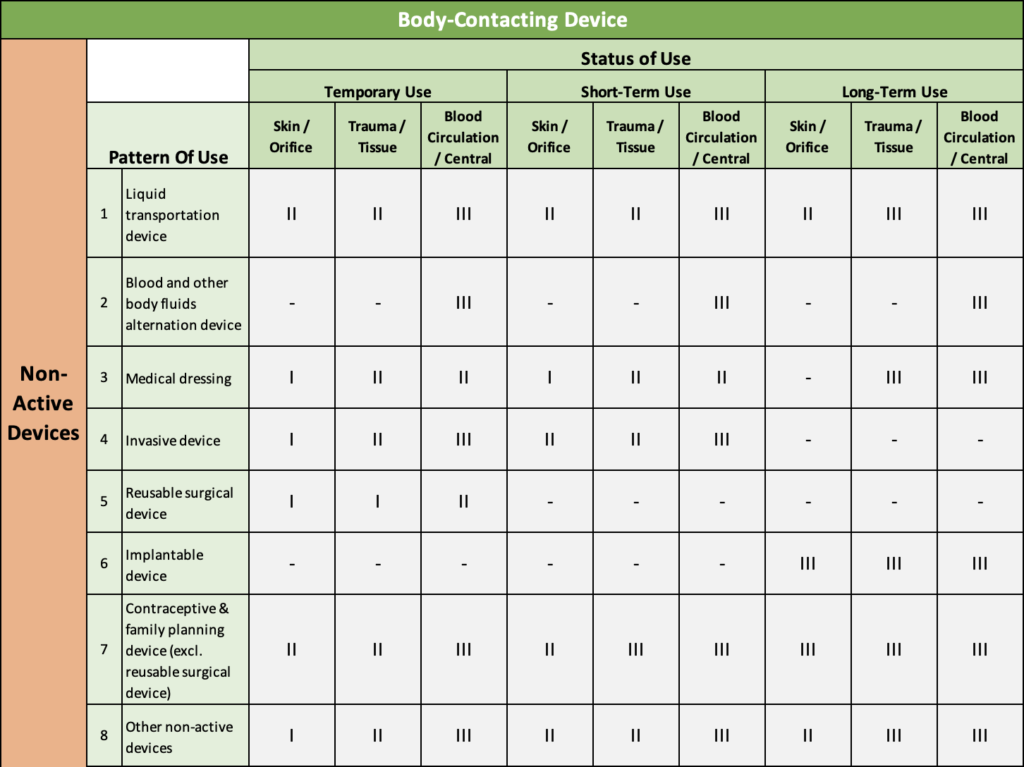
Table 2: Body Contacting & Active Devices
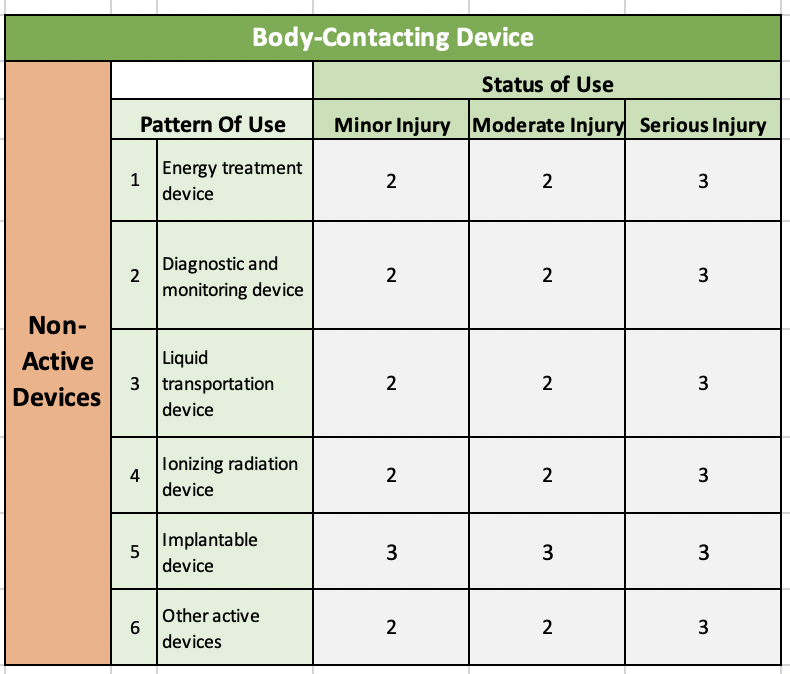
Table 3: Non-Body Contacting & Non-Active Devices
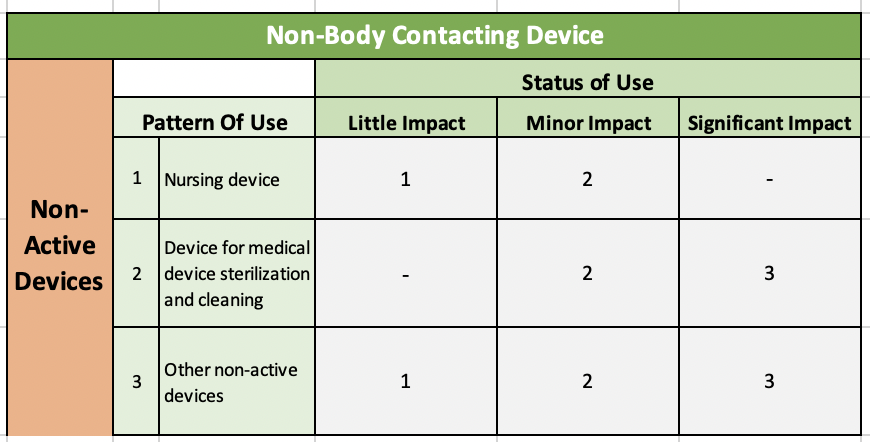
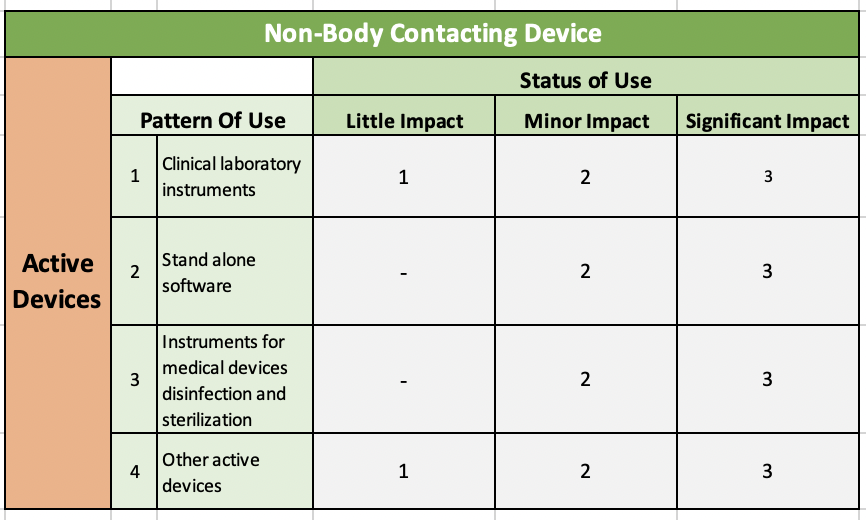
Table 4: Non-Body Contacting & Active Devices
Additional Requirements (Class II & III Devices)
Registering a class I device in China can be a relatively simple and straight forward affair.
But for class II and III medical devices, additional requirements are often demanded by the NMPA to register the product in China. These requirements include:
All class II and II devices must present proof of approval in their home country. This can include US 510(k), CE Mark, and ISO 13485.
Including detailed technical information, clinical data, & quality documentation.
Though some foreign test reports may be accepted, many additional tests must be conducted in China and according to Chinese applicable standards.
For many class III devices, additional animal and clinical trials will be required to be conducted in China.
Required to work on behalf of the device manufacturer to laisse with China’s medical device governing authorities.
Validation for class II and III devices are for 5 years and the renewal process must begin 6 months prior to the certificate expiry date.
Device Sub-Categories
Each medical device will also need to be assigned a category. Currently, there are 22 device categories in China. They include:
- Active Surgical Devices
- Passive Surgical Devices
- Neurological & Cardiovascular Surgical Devices
- Orthopedic Surgical Devices
- Radiotherapy Devices
- Medical Imaging Devices
- Medical Diagnostic & Monitoring Devices
- Respiratory, Anesthetic, & First-Aid Devices
- Physiotherapy Devices
- Blood Transfusion, Dialysis, & Extracorporeal Circulation Instruments
- Disinfection & Sterilization Devices of Medical Devices
- Active Implantable Devices
- Passive Implantable Devices
- Injection, Nursing, & Protection Devices
- Bed & Transportation Devices
- Ophthalmic Devices
- Dental Devices
- Gynecological & Obstetrical, Assisted Reproduction, & Contraceptive Devices
- Rehabilitation Devices
- Devices For Traditional Chinese Medicine
- Medical Software
- Clinical & Laboratory Instruments
How Kojima Can Help
Kojima help foreign medical device manufacturers navigate China’s registration landscape and obtain the necessary NMPA approvals to begin selling their products in China.
Kojima helps to:
- Define the device classification and category
- Formulate regulatory & registration strategy
- Determine what additional testing and clinical trials are needed
- Coordinate all required document translation & testing
- Prepare registration paperwork for final submission
- Handle any supplementary notice requests from NMPA




